For many individuals, regulating their blood pressure with medication is a daily routine that allows them to live a normal functioning life. What happens when that same medication may be doing you just as much harm as it is good?
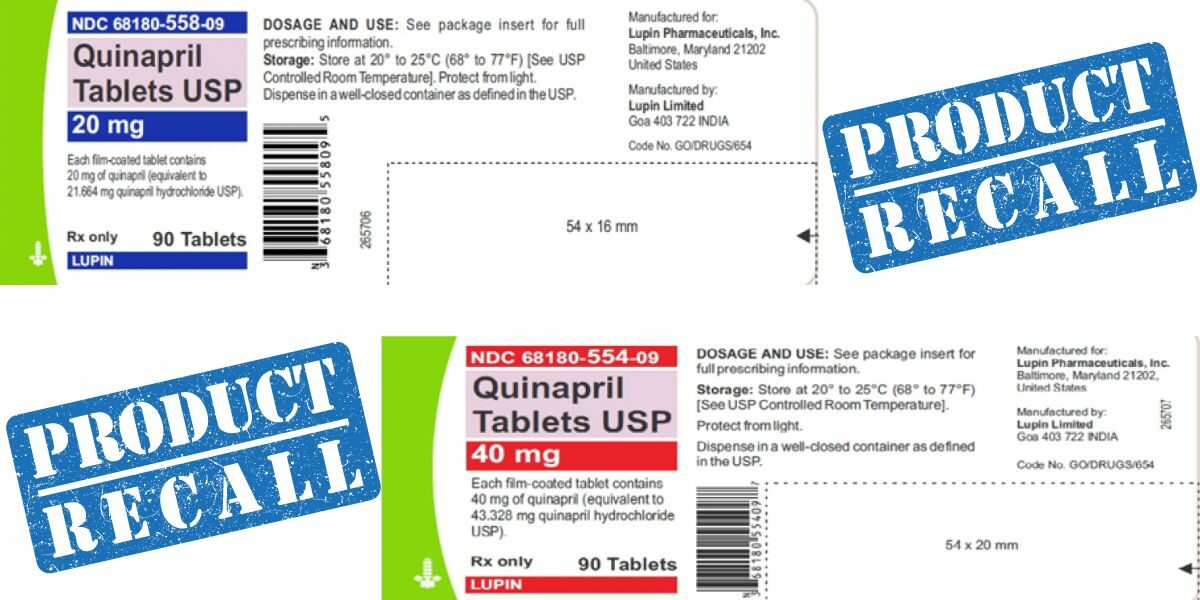
Lupin Pharmaceuticals Inc. is recalling four lots of their blood pressure medication Quinapril, due to the presence of a nitrosamine impurity, N-Nitroso-Quinapril, observed to be above the Acceptable Daily Intake (ADI) level.
Quinapril Tablets USP 20mg, and 40mg, packaged in 90 count bottles has been distributed nationwide in the U.S. The recalled lot numbers are G102929 for the 20mg tablets, and G100533, G100534, and G203071. The recall is due to the impurity being known to increase the risk of cancer.
For more information, visit: fda.gov.
Reporting for WGRT – Choze Powell